What Does the Kinetic Molecular Theory Describe
Describe the liquid state according to the kinetic-molecular theory. What is the theory used to.

Kinetic Molecular Theory Of Gases Practice Problems Youtube
The average kinetic energy of a collection of gas particles is directly proportional to absolute temperature only.
. It gives the relationship between pressure temperature and kinetic energy. The reactivity of valence electrons D. It gives the relationship between pressure temperature and kinetic energy.
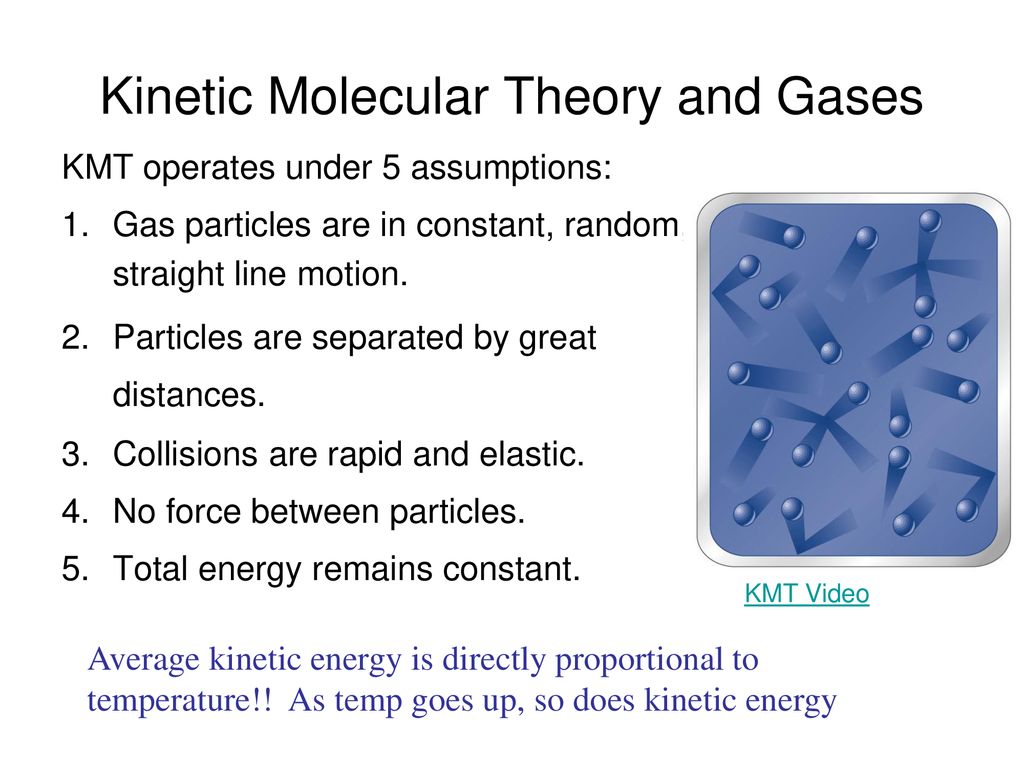
Under what conditions does a real gas most resemble an ideal gas. This can be explained using. Kinetic Molecular Theory states that gas particles are in constant motion and exhibit perfectly elastic collisions.
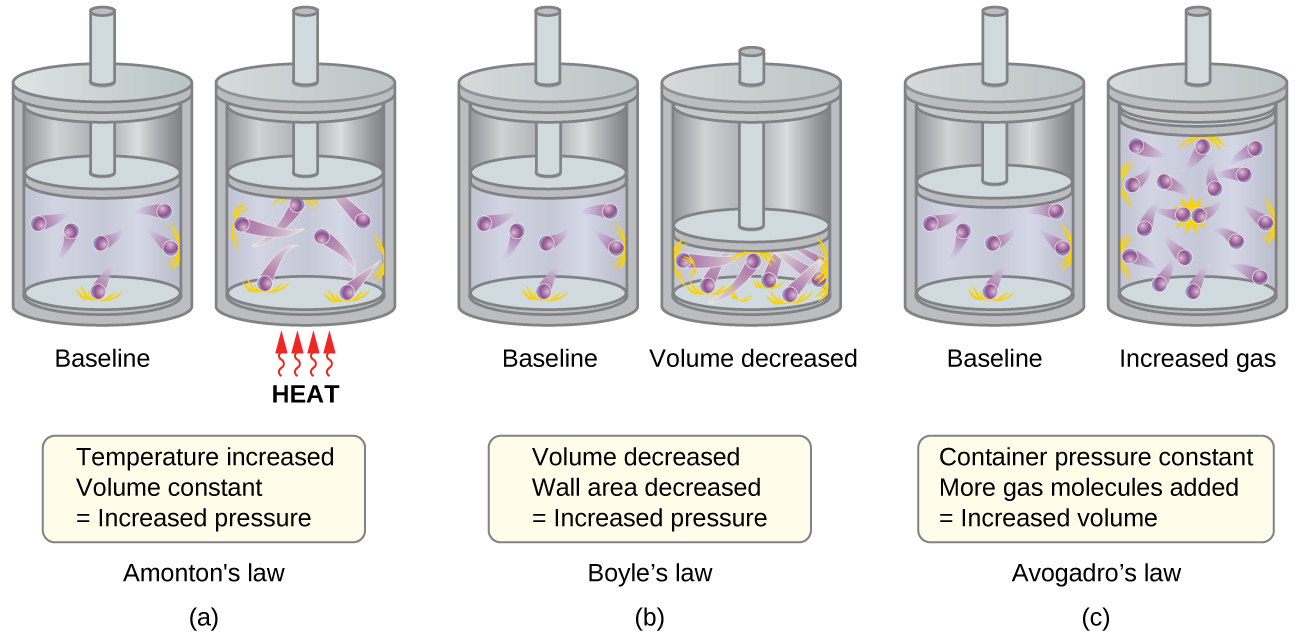
The kinetic molecular theory of matter states that. According to the kinetic-molecular theory of liquids the particles are not bound together in fixed positions. Gay-Lussacs Law states that in a closed system of fixed volume as the temperature of a gas increases the pressure increases as well.
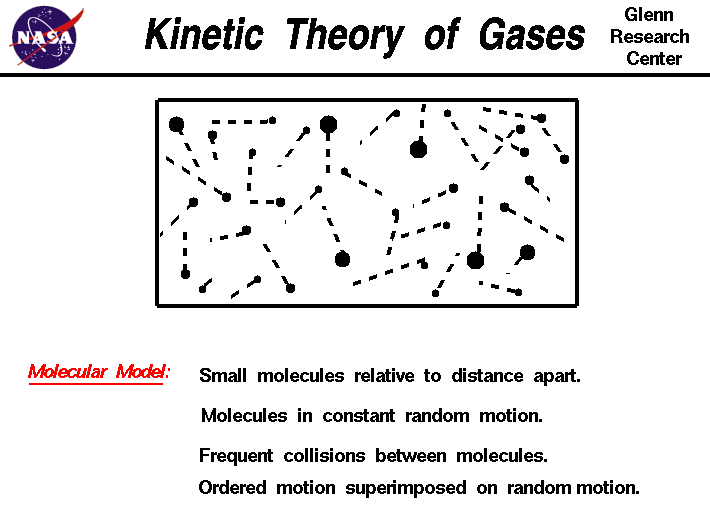
What does the kinetic theory describe. The kinetic molecular theory states that the motion of molecules is predictable based upon measurable traits such as the temperature volume and pressure of the atmosphere. Theory of treating samples of matter as a large number of small particles atoms or molecules all of which are in constant random motion.
Kinetic Molecular Theory can be used to explain both Charles and Boyles. Kinetic Molecular Theory can be used to explain both Charles and Boyles Laws. The Kinetic Molecular theory is used to describe the behavior of gas.
Properties of liquids include. Briefly state what the kinetic theory says. Definite volume but indefinite shape.
There is no area in between the specific particles so they can not compact. What does the kinetic theory describe. The kinetic-molecular theory explains the physical properties of solids liquids and gases in terms of the energy of particles and the A.
The Kinetic Molecular theory is used to describe the behavior of gas. The transfer of atoms in reactions B. The Kinetic Molecular Theory KMT is a model used to explain the behavior of matter.
The kinetic-molecular theory describes why gases are more compressible than either liquids or. Kinetic Molecular Theory can be used to explain both. The motion of atoms.
Using the kinetic molecular theory describe the three main ways that an ideal gas differs from a real gas 2. What is the theory used to. Kinetic Molecular Theory states that gas particles are in constant motion and exhibit perfectly elastic collisions.
Kinetic Molecular Theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. The energy of atomic orbitals C.
The Kinetic Molecular Theory Of Gases Fiveable
9 5 The Kinetic Molecular Theory Chemistry
Comments
Post a Comment